BACKGROUND
Given the poor prognosis and limited treatment options for patients with triple-class exposed/refractory multiple myeloma (TCE/R MM), there is a strong unmet need for novel interventions. Elranatamab, a B-cell maturation antigen (BCMA)- and CD3-directed bispecific antibody, recently demonstrated efficacy and safety in patients with TCE/R MM in the phase 2, single-arm MagnetisMM-3 trial (NCT04649359). The effectiveness of elranatamab was compared with physician's choice of treatment (PCT) in Canada through an unanchored matching-adjusted indirect comparison (MAIC), due to the absence of mature head-to-head comparative trial data.
METHODS
Individual patient data (IPD) from 14.7-month follow-up of MagnetisMM-3 (Cohort A [BCMA- naïve] N=123) was weighted to match published summary data from Canadian Myeloma Research Group (CMRG) database, which reported treatment patterns and outcomes on real-world patients with TCE/R MM in Canada (N = 199). Only BCMA-naïve patients from the MagnetisMM-3 trials were considered as BCMA therapies are not currently available as standard of care treatment options in Canada. To adjust for differences in baseline characteristics, patients from MagnetisMM-3 were reweighted to match those reported in the CRMG database. The adjustment variables were selected based on univariate Cox regressions using the MagnetisMM-3 IPD, a systematic literature review of prognostic variables and effect modifiers in relapsed or refractory MM, and a review of the recent analogous indirect comparisons, and confirmation by clinical experts.
Weights were determined using a propensity score-type logistic regression via the method of moments (Signorovitch et al. 2012), based on age, median time since diagnosis, and number of prior lines of therapy. In the analysis for the endpoint of overall survival (OS), sex was also included in the analysis. A limitation of this MAIC is that key prognostic variables, such as International Staging System disease stage, cytogenetic risk, and extramedullary disease, were not adjusted for in the analysis as the definitions were not comparable between the data sources.
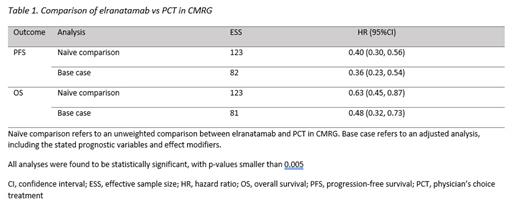
Unanchored MAIC analyses used R code provided in the National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) 18 by Phillippo et al (2016). The respective outcomes in the MAIC were OS and progression-free survival (PFS) based on the 15-month follow-up data for elranatamab. Results were reported as hazard ratios (HRs) for time-to-event endpoints, with 95% confidence intervals (CIs).
RESULTS
Median PFS and OS was not reached in the MagnetisMM-3 trial. Patients with TCE/RMM treated with PCT had a median PFS of 4.4 months (95% CI 3.6 - 5.3) and a median OS of 10.5 months (95% CI 8.5 - 13.8). Following weighting, the summary statistics of key baseline characteristics were matched between elranatamab and CMRG. The effective sample size was 82 post-matching for PFS and 81 post-matching for OS. Compared with the patients in the CMRG database, elranatamab was associated with better PFS (HR: 0.36; 95% CI 0.23 - 0.54) and better OS (HR: 0.48; 95% CI 0.32 - 0.73).
CONCLUSIONS
In this MAIC, among TCE/R MM patients elranatamab demonstrated a significantly longer OS and PFS compared with PCT in the real-world setting reported in the CMRG database.
Disclosures
Mol:Pfizer Inc: Consultancy. Hu:Pfizer Inc: Consultancy. Fanton-Aita:Pfizer Canada ULC: Current Employment. On:Pfizer Canada ULC: Current Employment. Gonzalez:Pfizer Canada ULC: Current Employment. LeBlanc:Janssen: Honoraria, Other: Participation to Advisory Board ; BMS: Other: Participation to Advisory Board ; Amgen: Other: Participation to Advisory Board , Research Funding; Sanofi: Other: Participation to Advisory Board , Research Funding; FORUS Therapeutics: Other: Participation to Advisory Board ; Pfizer: Honoraria. Reece:GSK: Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Honoraria; BMS: Consultancy, Honoraria, Research Funding; Sanofi: Honoraria; Amgen: Consultancy; Millennium: Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Visram:Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Apotex: Consultancy, Honoraria. Cappelleri:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Chu:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Nador:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Hlavacek:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal